DISCOVER
INVESTIGATOR SITE MANAGEMENT

Clinical Trials Mobile Application
We provide innovative digital solutions aiming at facilitating patient recruitment in clinical studies, using current patients’ conditions and powerful build-in algorithms.
Get a demoOur mission
SOME NUMBERS
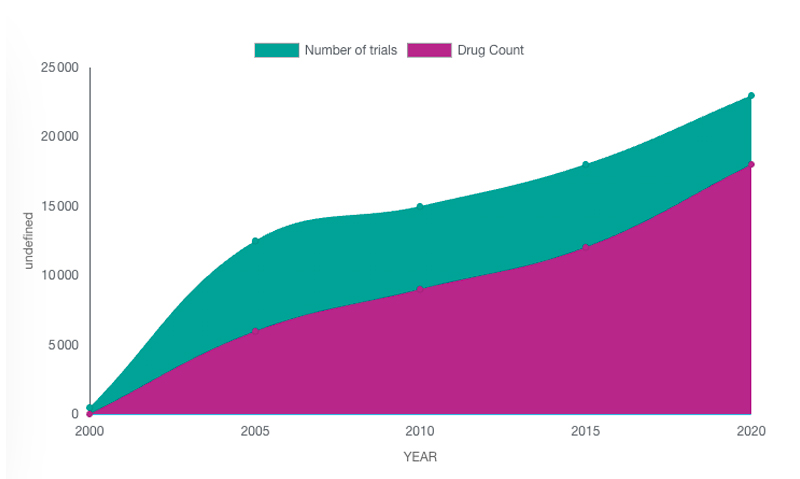
Figure: Total R&D/clinical trials pipeline size, by year 2000-2022
They are so many drugs in development that it is extremely challenging to finds the way to one of them, as a patient or as a site, or to secure a high visibility on one of them for a Sponsor.
CT-SCOUT™ and ToTem4me facilitate the match between the patients and the studies.

50%
OF STUDIES FAIL TO MEET PATIENT RECRUITMENT DEADLINES
However, millions of patients visit clinical sites every day.
CT-SCOUT™ help investigators identify the ones that could benefit from a clinical study.

80%
OF THE PATIENTS WOULD WELCOME THE OPPORTUNITY TO PARTICIPATE TO A CLINICAL STUDY
Patients have become actors of their treatment conditions.
ToTem4me help them identify potential opportunities through clinical studies.
0.02%
OF THE PATIENTS ARE BEING OFFERED TO PARTICIPATE TO A CLINICAL STUDY
But 80% of patients would accept to participate t a clinical study.
CT-SCOUT™ contributes to increase the percentage of patients considered for clinical research.

2
WEEKS SET-UP TIME
No more than 2 weeks are required to get CT-SCOUT™
or ToTem4me deployed for a clinical study
Our Solutions
We have customised digital solutions for the different contributors to the clinical research efforts.

CT-SCOUT™
The missing link between sponsor & sites
A multi-device application based on a unique algorithmic model to enable all physicians to quickly detect in real-time, the clinical studies running at their sites, for which a patient is potentially eligible according to his/her current health conditions.
Find out more


eISF
The electronic investigator site file
The eISF, or electronic Investigator Site File, is the digital version of the paper-based investigator site file (ISF). It enables the collection in digital form of essential documents that demonstrate a clinical trial was conducted in accordance with Good Clinical Practice (GCP) guidelines, as well as regulatory and sponsor requirements.
Find out more

LATEST NEWSOUR NEWS >
January 29, 2026
Medtech Dive Amid a deregulatory push by the Trump administration, the Food and Drug Administration is scrutinizing its digital health policies. The agency suddenly issued a pair of guidances earlier this month, intended to clarify its approach to wellness devices and medical software. The updates reflect changes to the agency’s thinking about what counts as a wellness […]
January 29, 2026
Med City News The Covid-19 pandemic impacted virtually every industry, with healthcare arguably undergoing the most drastic transformation. Pre-pandemic, most of us never could’ve imagined routine doctor visits happening over telehealth, or a brand-new vaccine being developed, tested, and authorized for emergency use in under a year. The urgency of the pandemic forced medical scientists […]
January 29, 2026
Med City News Digital databases for clinical trial searches have already changed healthcare accessibility, bringing critical information about clinical trials to the patients who need it – and to the physicians who care for them. But these databases are also enormous – alone has details of close to 500,000 studies. As a result, information about […]









