DISCOVER
INVESTIGATOR SITE MANAGEMENT

Clinical Trials Mobile Application
We provide innovative digital solutions aiming at facilitating patient recruitment in clinical studies, using current patients’ conditions and powerful build-in algorithms.
Get a demoOur mission
SOME NUMBERS
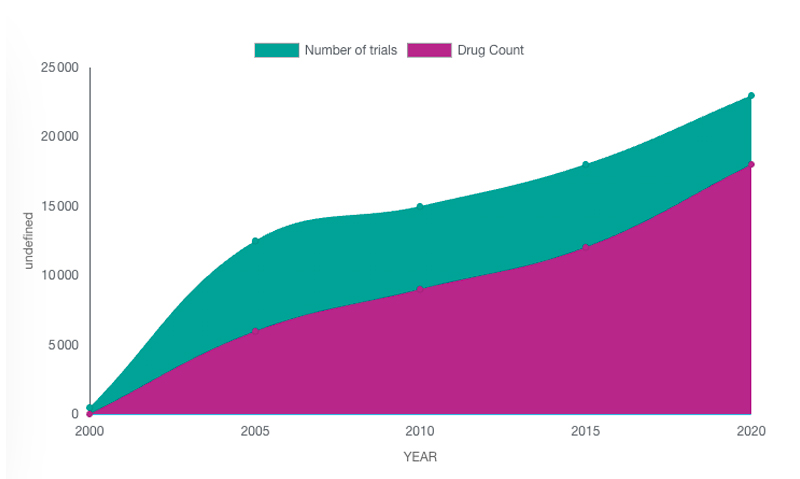
Figure: Total R&D/clinical trials pipeline size, by year 2000-2022
They are so many drugs in development that it is extremely challenging to finds the way to one of them, as a patient or as a site, or to secure a high visibility on one of them for a Sponsor.
CT-SCOUT™ and ToTem4me facilitate the match between the patients and the studies.

50%
OF STUDIES FAIL TO MEET PATIENT RECRUITMENT DEADLINES
However, millions of patients visit clinical sites every day.
CT-SCOUT™ help investigators identify the ones that could benefit from a clinical study.

80%
OF THE PATIENTS WOULD WELCOME THE OPPORTUNITY TO PARTICIPATE TO A CLINICAL STUDY
Patients have become actors of their treatment conditions.
ToTem4me help them identify potential opportunities through clinical studies.
0.02%
OF THE PATIENTS ARE BEING OFFERED TO PARTICIPATE TO A CLINICAL STUDY
But 80% of patients would accept to participate t a clinical study.
CT-SCOUT™ contributes to increase the percentage of patients considered for clinical research.

2
WEEKS SET-UP TIME
No more than 2 weeks are required to get CT-SCOUT™
or ToTem4me deployed for a clinical study
Our Solutions
We have customised digital solutions for the different contributors to the clinical research efforts.

CT-SCOUT™
The missing link between sponsor & sites
A multi-device application based on a unique algorithmic model to enable all physicians to quickly detect in real-time, the clinical studies running at their sites, for which a patient is potentially eligible according to his/her current health conditions.
Find out more


eISF
The electronic investigator site file
The eISF, or electronic Investigator Site File, is the digital version of the paper-based investigator site file (ISF). It enables the collection in digital form of essential documents that demonstrate a clinical trial was conducted in accordance with Good Clinical Practice (GCP) guidelines, as well as regulatory and sponsor requirements.
Find out more

LATEST NEWSOUR NEWS >
June 26, 2025
June 30 – 4:00 PM to 5:15 PM (CET) xShare, an EU-funded project, strengthens procurement capabilities in digital health by translating the European Electronic Health Record Exchange Format (EEHRxF) into concrete requirements for tenders. This inaugural webinar presents key EU rules and promotes the procurement of interoperable solutions. It aims to raise awareness, share innovative […]
June 26, 2025
June 25–27 – JETRO – Japan External Trade Organization Pavilion – Osaka Booth Hall 6‑A, A‑0408 Philippe Haran – MD, is representing Telemedicine Technologies at the Japan Health Exhibition 2025, as a guest of Micron, Inc., a valued member of our CROAlliance partner. At Micron’s booth, he’s engaging with healthcare leaders and exploring collaboration opportunities […]
June 26, 2025
Applied Clinical Trials Online At a time when artificial intelligence (AI) is redefining industries at an unprecedented pace, the pharmaceutical sector stands on the brink of a progressive transformation. AI is not just a tool but a catalyst that promises to accelerate drug development timelines in ways we’ve never seen before. However, the true potential […]









