DISCOVER
CRO ALLIANCE
The power
of an international network
The CRO Alliance is an international network of CROs sharing the same technological platform for clinical trials: CleanWeb.

This Alliance enables:
- Perfect match with individual CRO partner current resources and services
- Significant competitive advantage on the CRO partner respective market(s)
- Set up of all clinical trial phases and complexity
- Access to TECH Alliance innovations
- Dedicated support of the CRO partner to boost business growth with a single point-of contact
- Provision of a DPO for all CRO Alliance members
- Global contacts database to increase business opportunities
A FULL SERVICE OFFER
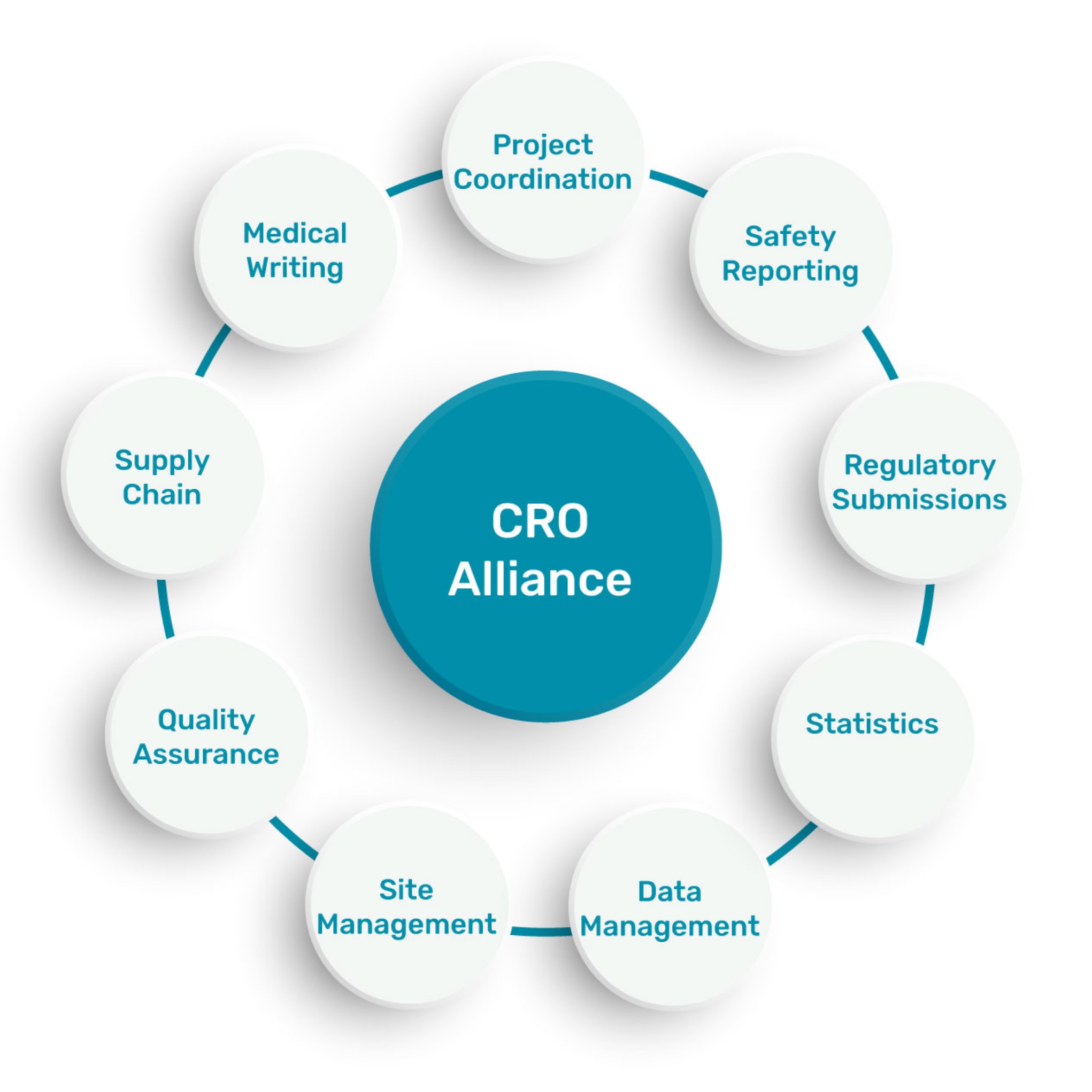
A network of CROs to offer full services
in an international scale:
OUR COMMITMENTS
- Commitment to prioritise preparation of proposals for the CROs in the Alliance
- Detailed proposal matching of study-specific needs in up to 1 week
- Availability for demos, F2F meetings and teleconferences to support the CRO partner during its interactions with Sponsors, under CRO partner umbrella
- Willingness to evaluate eClinical tools upgrades matching CRO partners’ needs & processes
22 years of experience
All phases
Full Flexibility
Multiple user friendly tools and apps, combined or stand-alone, in interaction with externel data sources and systems or not
Very strong focus on Quality
Combined expertise in eClinical as CRO
Cost-effectiveness model
Collaborative ways of working
Built according to individual cro partner needs
Interested in becoming part of the CRO Alliance or just want to learn more?
A LEADING POSITION AND
AN INTERNATIONAL DEPLOYMENT
64 +
countries
involved
20 000 +
investigational
Centres
3 million +
patients
in database
50 000 +
users
worlwide

LATEST NEWSOUR NEWS >
June 27, 2024
25 September 2024 from 4:00PM to 5:30PM (CEST) Webinar Overview It has been demonstrated that using standards from the start of clinical research studies (i.e. protocol and CRF development) can save significant time and resources and results in higher quality research. Unfortunately, it is not always clear how to select the appropriate standards for the […]
June 27, 2024
Applied Clinical Trials Online ACT: How can data management challenges be addressed? Specifically, is there any opportunity to address them with artificial intelligence (AI)? Lacroix: There is a lot of opportunity across the life cycle to assist with this and it’s interesting because historically it was always: we want to throw people at some of […]
June 27, 2024
Fiercebiotech Experts recommend that CGT manufacturers proactively design a comprehensive market access strategy early in clinical development and commercial planning timelines, ideally more than three years prior to launch. This strategy should focus on addressing the nuanced market access challenges for such treatments, including but not limited to, distribution network design, tailored commercial site engagement, […]


















