Axonal Biostatem

For more than 30 years, AXONAL BIOSTATEM supported their clients in the design, implementation and analysis of their clinical research projects and non-interventional studies. While offering a European and international coverage, their medical and scientific expertise as well as their ability to adapt to the specificities of each project, helped them become a solid and sustainable company.
Their main goals consist of supporting medical research stakeholders to obtain reliable and honest data, facilitating early patient access to innovative therapies and reducing the environmental impact of their activities.
Learn more: www.axonalbiostatem.com
A LEADING POSITION AND
AN INTERNATIONAL DEPLOYMENT
64 +
countries
involved
20 000 +
investigational
Centres
3 million +
patients
in database
50 000 +
users
worlwide

LATEST NEWSOUR NEWS >
December 13, 2025
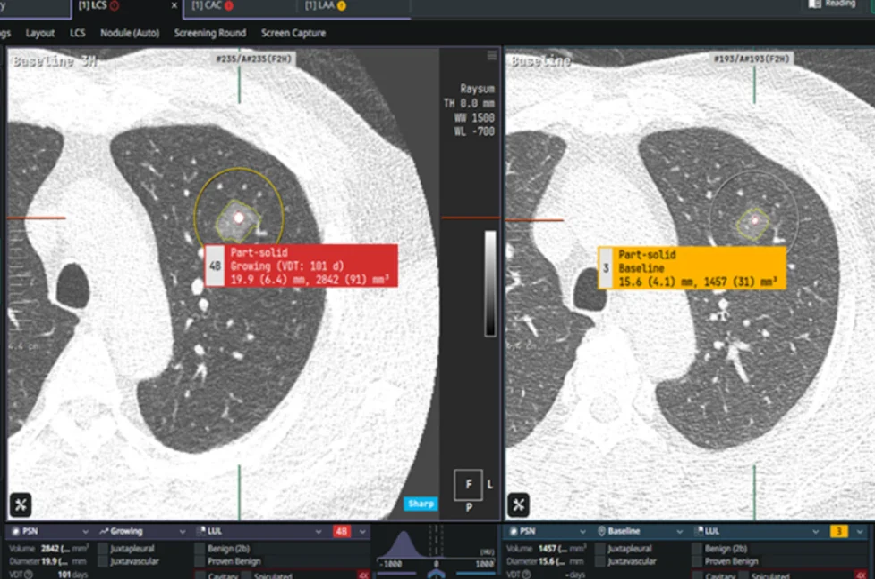
Mind Health With 53,000 new cases diagnosed each year, lung cancer is the deadliest cancer in France, according to data from the National Cancer Institute. Three-quarters of cases are detected at an advanced stage, which reduces the chances of recovery. The importance of screening is therefore crucial. In this field, the combination of low-dose CT […]
November 27, 2025
Med City News Healthcare quality isn’t improving at scale, despite advances in technology, because many systems treat it as another compliance task rather than a priority. Stephanie Mercado, CEO of the National Association for Healthcare Quality (NAHQ), argued that investing in workforce skills and standardizing roles can drive safer, higher-quality care while delivering measurable cost […]
November 27, 2025
Trial Site News Clinical trial reporting of treatment efficacy is the foundation of public trust in medicine. Yet pharmaceutical companies often report efficacy measured as a relative risk reduction (RRR) while omitting the more relevant absolute risk reduction (ARR). This statistical sleight of hand can make clinically insignificant benefits look spectacular! Consider a simple example A risk is […]