DISCOVER
REGISTRIES
REGISTRY OF PRACTICES, OBSERVATIONAL STUDIES, DATABASE
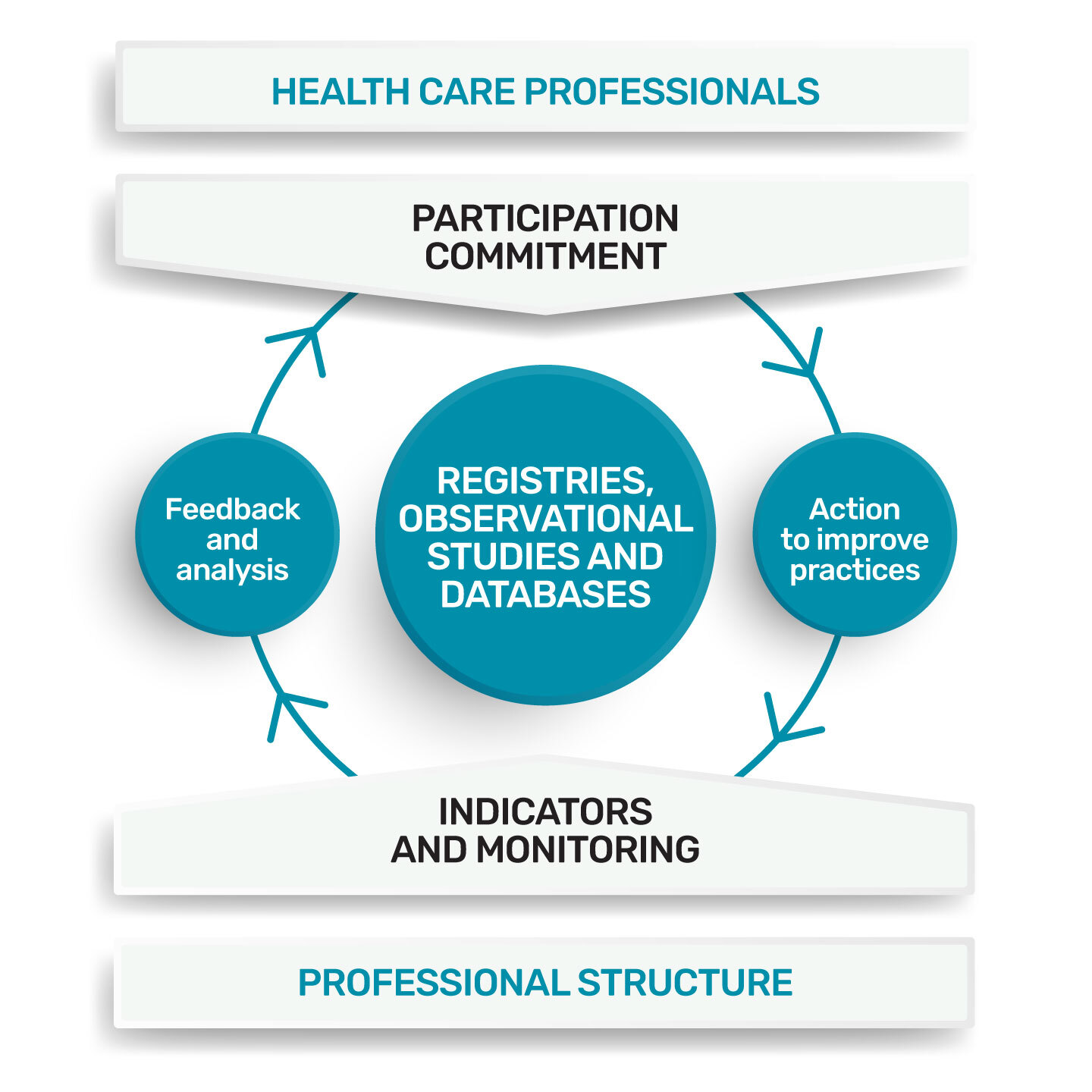
The collection and analysis of standardised data from professional practices are particularly widespread within professional organisations, learned societies and networks. The organisation and content of these collections can take various forms: registry, observational studies, database. Integrated for the purpose of improving practices, these programmes constitute a CPD method. (Continuing Professional Development)


HEALTH
PROFESSIONALS
AND PROFESSIONAL STRUCTURES
Telemedicine Technologies supports HCPs (Healthcare Professionals) and PSs (Professional Structures), with the technical implementation of these databases, as well as monitoring indicators and feedback to the actors of this registry (HCPs, PSs).


The registry incorporating the improvement process is set up and managed by a professional structure, made up of peers, this structure is responsible for:
• the theme (professional practices);
• the design of the approach;
• the quality of the data collected;
• the analysis and use of these practices.
The indicators put in place, targeted at key elements of practice and easy to collect, make it possible to objectify the quality of care and to monitor the improvement initiatives undertaken. Thus, in Data Visualisation, we can restore these indicators, by adding a comparative component. For example, each PDS can compare his practice with those of all the PDSs in his organisation or with the average practice of all the PDSs in his speciality, and more generally, with the whole practice of the PDS of a registry in a European registry.
EUROPEAN
REGISTRIES
Telemedicine Technologies also participates in the technical support of structures wishing to create European registries based on national registries existing in different countries, to create a common register with a minimum of information that is specific to each country and making it possible to centralise information from these different countries in a database, independent of each national registry and supplied from these national registries by interoperability with the desired update frequency.
Our Data Services structure can also offer the setup of European databases in CDISC format, to allow and facilitate exchanges with regulatory agencies.

A LEADING POSITION AND
AN INTERNATIONAL DEPLOYMENT
64 +
countries
involved
20 000 +
investigational
Centres
3 million +
patients
in database
50 000 +
users
worlwide

LATEST NEWSOUR NEWS >
February 26, 2026
During the EUCROF 2026 gala dinner in Amsterdam, in front of 300+ participants from the European clinical research ecosystem, the xShare project Open Call Awards for Clinical Research were officially presented. We are extremely proud that Telemedicine Technologies contributed to three complementary xShare Open Call projects (out of 10), all recognised for their quality, innovation […]
January 29, 2026
Medtech Dive Amid a deregulatory push by the Trump administration, the Food and Drug Administration is scrutinizing its digital health policies. The agency suddenly issued a pair of guidances earlier this month, intended to clarify its approach to wellness devices and medical software. The updates reflect changes to the agency’s thinking about what counts as a wellness […]
January 29, 2026
Med City News The Covid-19 pandemic impacted virtually every industry, with healthcare arguably undergoing the most drastic transformation. Pre-pandemic, most of us never could’ve imagined routine doctor visits happening over telehealth, or a brand-new vaccine being developed, tested, and authorized for emergency use in under a year. The urgency of the pandemic forced medical scientists […]




