DISCOVER
CT-SCOUT™

Success is when the time to access an innovative treatment is reduced.
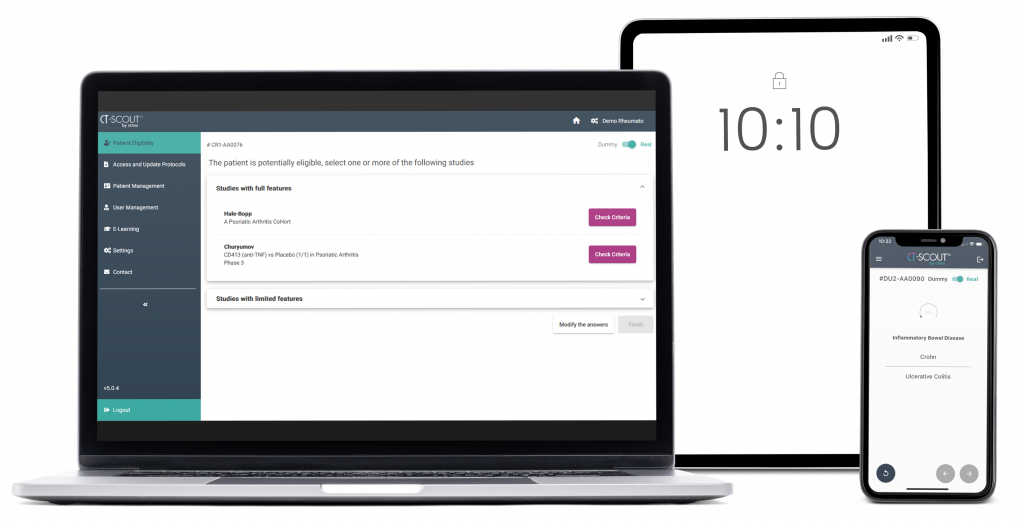
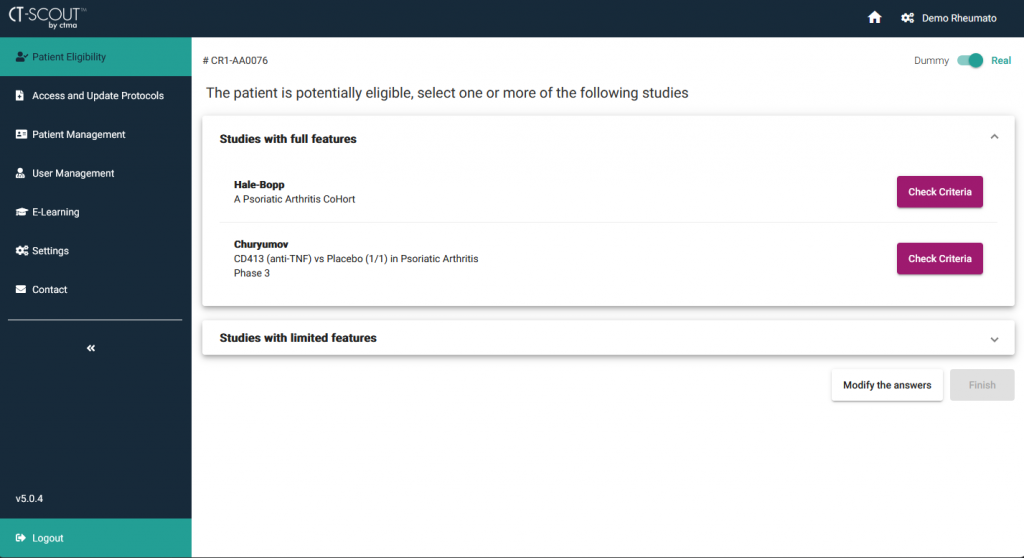
CT-SCOUT™ is a multi-device application aiming at facilitating the recruitment of patients in clinical studies...
It assists the investigational team in detecting the studies for which a patient is potentially eligible according to his/her current health conditions.
The digital solution to boost patient recruitment in clinical trials
Transforms all physician into real time recruiters
Anywhere, anytime
On any device
Easy to use
CT-SCOUT™ unique features
1. An efficient algorithmic model
2. Real-time detection
3. Simplified
process
Therapeutic Expertise
Gastroenterology
Crohn disease
Ulcerative colitis
Dermatology
Psoriasis
Atopic dermatitis
Hidradenitis suppurativa
Rheumatology
Rheumatoid arthritis
Ankylosing spondylitis
Psoriatic arthritis
200 +
studies registered (industrial & academic)
1000 +
users registered
170 +
centers in various countries (Belgium, Canada, France, Germany, Israel, Italy, Netherlands, Spain, Switzerland, UK…)
7000 +
Patients detected
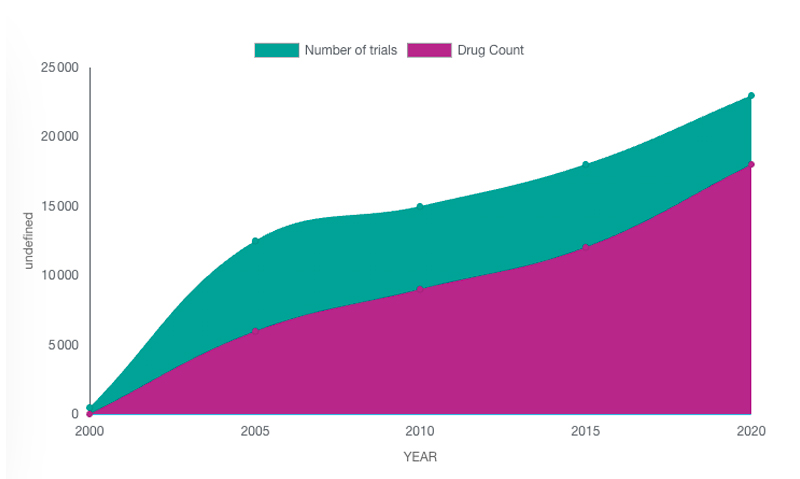
Figure: Total R&D/clinical trials pipeline size, by year 2000-2022
They are so many drugs in development that it is extremely challenging to finds the way to one of them, as a patient or as a site, or to secure a high visibility on one of them for a Sponsor. CT-SCOUT™and ToTem4me facilitate the match between the patients and the studies.

50%
OF STUDIES FAIL TO MEET PATIENT RECRUITMENT DEADLINES
However, millions of patients visit clinical sites every day.
CT-SCOUT™ help investigators identify the ones that could benefit from a clinical study.

80%
OF THE PATIENTS WOULD WELCOME THE OPPORTUNITY TO PARTICIPATE TO A CLINICAL STUDY
Patients have become actors of their treatment conditions.
ToTem4me help them identify potential opportunities through clinical studies.
0.02%
OF THE PATIENTS ARE BEING OFFERED TO PARTICIPATE TO A CLINICAL STUDY
But 80% of patients would accept to participate t a clinical study.
CT-SCOUT™ contributes to increase the percentage of patients considered for clinical research.

2
WEEKS SET-UP TIME
No more than 2 weeks are required to get CT-SCOUT™
or ToTem4me deployed for a clinical study
As a summary
Assist investigational teams in their clinical research activities.
Shorten clinical trials by boosting detection of patients potentially eligible to clinical trials.
Allow patients’ earlier access to innovative and life-saving treatment.
LATEST NEWSOUR NEWS >
February 26, 2026
During the EUCROF 2026 gala dinner in Amsterdam, in front of 300+ participants from the European clinical research ecosystem, the xShare project Open Call Awards for Clinical Research were officially presented. We are extremely proud that Telemedicine Technologies contributed to three complementary xShare Open Call projects (out of 10), all recognised for their quality, innovation […]
January 29, 2026
Medtech Dive Amid a deregulatory push by the Trump administration, the Food and Drug Administration is scrutinizing its digital health policies. The agency suddenly issued a pair of guidances earlier this month, intended to clarify its approach to wellness devices and medical software. The updates reflect changes to the agency’s thinking about what counts as a wellness […]
January 29, 2026
Med City News The Covid-19 pandemic impacted virtually every industry, with healthcare arguably undergoing the most drastic transformation. Pre-pandemic, most of us never could’ve imagined routine doctor visits happening over telehealth, or a brand-new vaccine being developed, tested, and authorized for emergency use in under a year. The urgency of the pandemic forced medical scientists […]



