DISCOVER
CRO ALLIANCE
The power
of an international network
The CRO Alliance is an international network of CROs sharing the same technological platform for clinical trials: CleanWeb.

This Alliance enables:
- Perfect match with individual CRO partner current resources and services
- Significant competitive advantage on the CRO partner respective market(s)
- Set up of all clinical trial phases and complexity
- Access to TECH Alliance innovations
- Dedicated support of the CRO partner to boost business growth with a single point-of contact
- Provision of a DPO for all CRO Alliance members
- Global contacts database to increase business opportunities
A FULL SERVICE OFFER
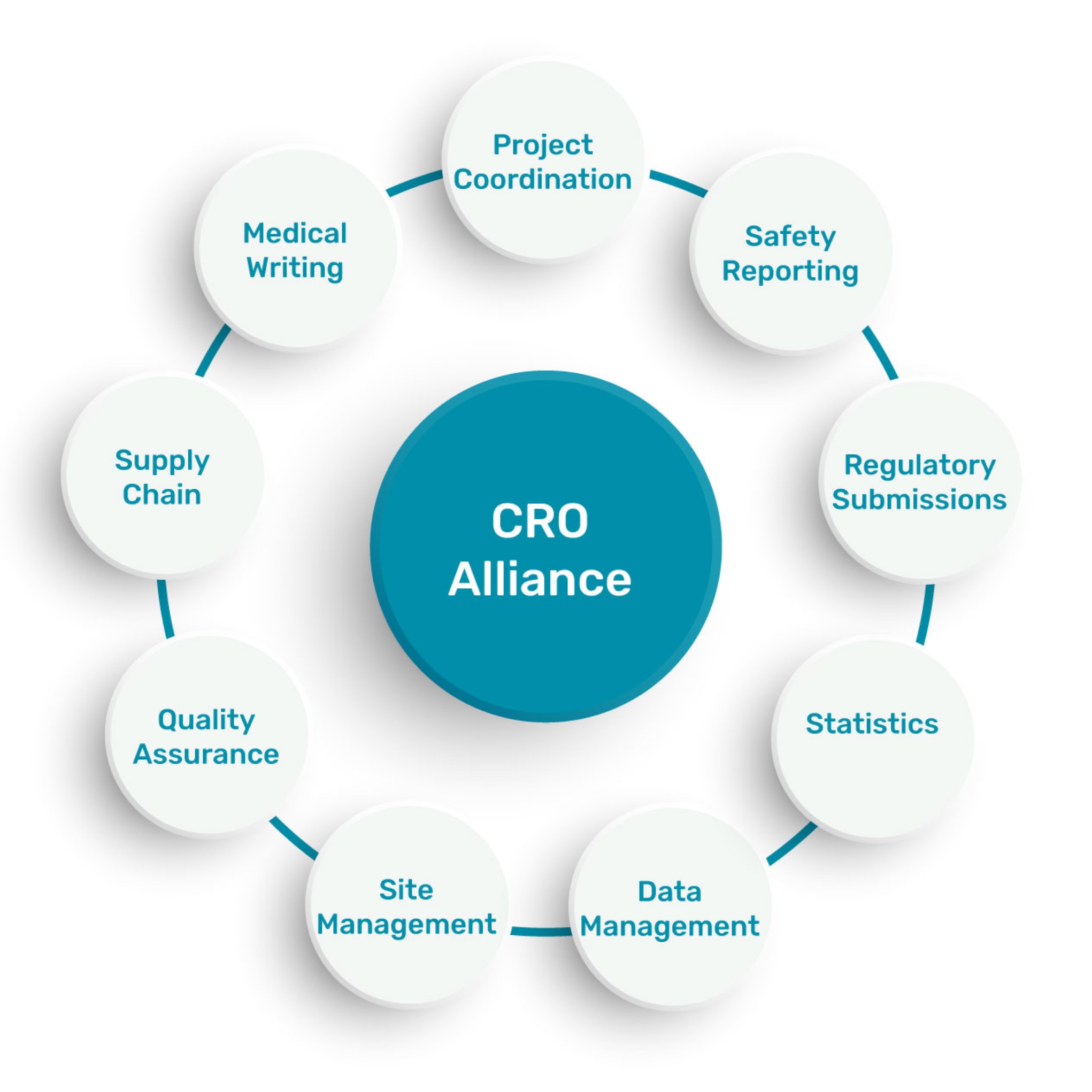
A network of CROs to offer full services
in an international scale:
OUR COMMITMENTS
- Commitment to prioritise preparation of proposals for the CROs in the Alliance
- Detailed proposal matching of study-specific needs in up to 1 week
- Availability for demos, F2F meetings and teleconferences to support the CRO partner during its interactions with Sponsors, under CRO partner umbrella
- Willingness to evaluate eClinical tools upgrades matching CRO partners’ needs & processes
22 years of experience
All phases
Full Flexibility
Multiple user friendly tools and apps, combined or stand-alone, in interaction with externel data sources and systems or not
Very strong focus on Quality
Combined expertise in eClinical as CRO
Cost-effectiveness model
Collaborative ways of working
Built according to individual cro partner needs
Interested in becoming part of the CRO Alliance or just want to learn more?
A LEADING POSITION AND
AN INTERNATIONAL DEPLOYMENT
64 +
countries
involved
20 000 +
investigational
Centres
3 million +
patients
in database
50 000 +
users
worlwide

LATEST NEWSOUR NEWS >
February 26, 2026
During the EUCROF 2026 gala dinner in Amsterdam, in front of 300+ participants from the European clinical research ecosystem, the xShare project Open Call Awards for Clinical Research were officially presented. We are extremely proud that Telemedicine Technologies contributed to three complementary xShare Open Call projects (out of 10), all recognised for their quality, innovation […]
January 29, 2026
Medtech Dive Amid a deregulatory push by the Trump administration, the Food and Drug Administration is scrutinizing its digital health policies. The agency suddenly issued a pair of guidances earlier this month, intended to clarify its approach to wellness devices and medical software. The updates reflect changes to the agency’s thinking about what counts as a wellness […]
January 29, 2026
Med City News The Covid-19 pandemic impacted virtually every industry, with healthcare arguably undergoing the most drastic transformation. Pre-pandemic, most of us never could’ve imagined routine doctor visits happening over telehealth, or a brand-new vaccine being developed, tested, and authorized for emergency use in under a year. The urgency of the pandemic forced medical scientists […]




















