DISCOVER
CRO ALLIANCE
The power
of an international network
The CRO Alliance is an international network of CROs sharing the same technological platform for clinical trials: CleanWeb.

This Alliance enables:
- Perfect match with individual CRO partner current resources and services
- Significant competitive advantage on the CRO partner respective market(s)
- Set up of all clinical trial phases and complexity
- Access to TECH Alliance innovations
- Dedicated support of the CRO partner to boost business growth with a single point-of contact
- Provision of a DPO for all CRO Alliance members
- Global contacts database to increase business opportunities
A FULL SERVICE OFFER
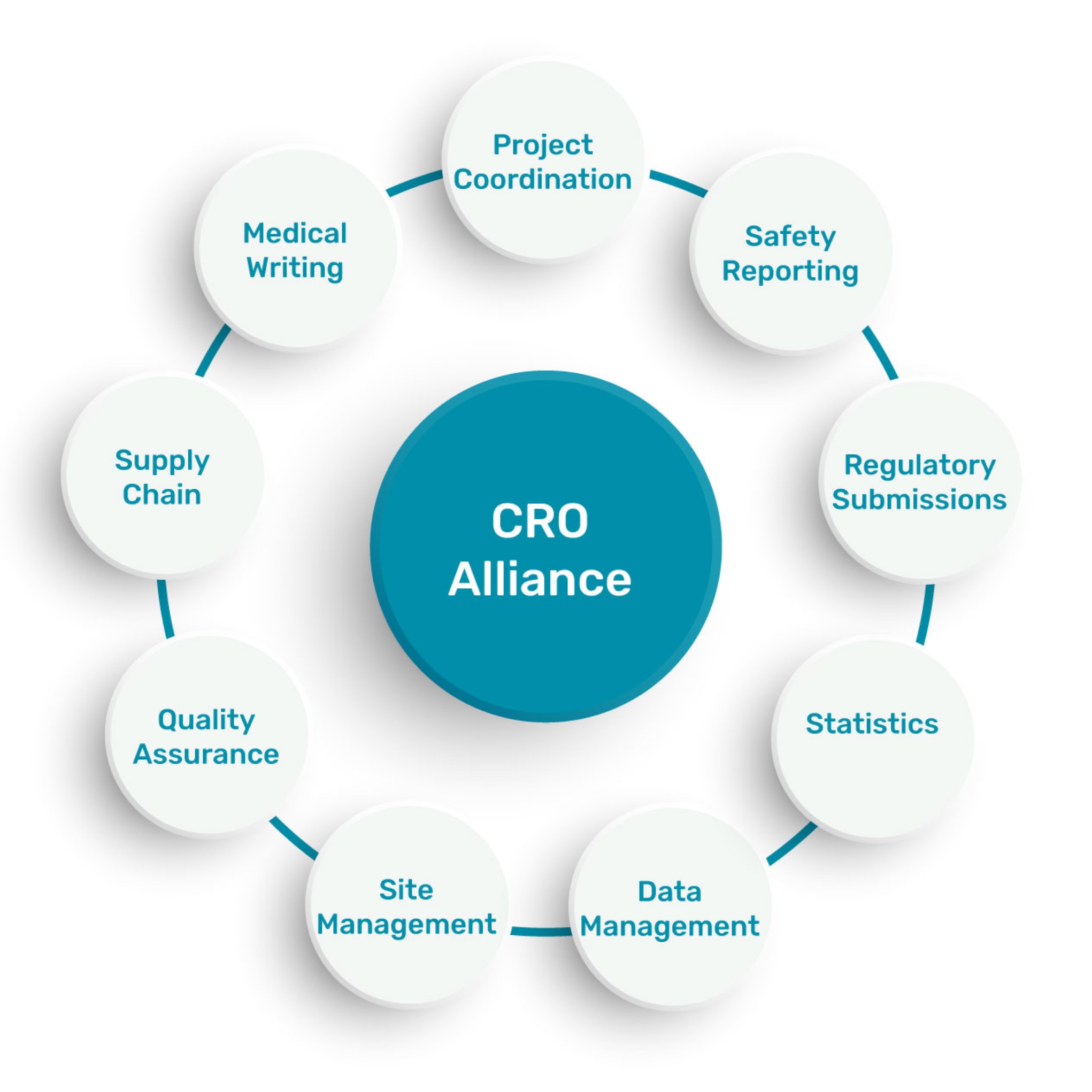
A network of CROs to offer full services
in an international scale:
OUR COMMITMENTS
- Commitment to prioritise preparation of proposals for the CROs in the Alliance
- Detailed proposal matching of study-specific needs in up to 1 week
- Availability for demos, F2F meetings and teleconferences to support the CRO partner during its interactions with Sponsors, under CRO partner umbrella
- Willingness to evaluate eClinical tools upgrades matching CRO partners’ needs & processes
22 years of experience
All phases
Full Flexibility
Multiple user friendly tools and apps, combined or stand-alone, in interaction with externel data sources and systems or not
Very strong focus on Quality
Combined expertise in eClinical as CRO
Cost-effectiveness model
Collaborative ways of working
Built according to individual cro partner needs
Interested in becoming part of the CRO Alliance or just want to learn more?
A LEADING POSITION AND
AN INTERNATIONAL DEPLOYMENT
64 +
countries
involved
20 000 +
investigational
Centres
3 million +
patients
in database
50 000 +
users
worlwide

LATEST NEWSOUR NEWS >
December 13, 2025
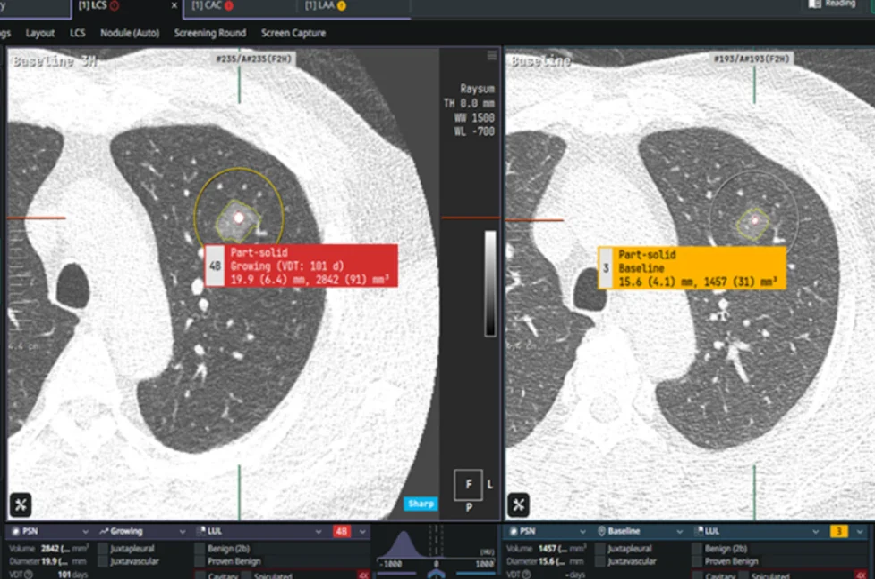
Mind Health With 53,000 new cases diagnosed each year, lung cancer is the deadliest cancer in France, according to data from the National Cancer Institute. Three-quarters of cases are detected at an advanced stage, which reduces the chances of recovery. The importance of screening is therefore crucial. In this field, the combination of low-dose CT […]
November 27, 2025
Med City News Healthcare quality isn’t improving at scale, despite advances in technology, because many systems treat it as another compliance task rather than a priority. Stephanie Mercado, CEO of the National Association for Healthcare Quality (NAHQ), argued that investing in workforce skills and standardizing roles can drive safer, higher-quality care while delivering measurable cost […]
November 27, 2025
Trial Site News Clinical trial reporting of treatment efficacy is the foundation of public trust in medicine. Yet pharmaceutical companies often report efficacy measured as a relative risk reduction (RRR) while omitting the more relevant absolute risk reduction (ARR). This statistical sleight of hand can make clinically insignificant benefits look spectacular! Consider a simple example A risk is […]