DISCOVER
trainings
Our training organisation
is Qualiopi certified…
…a new quality guarantee for training providers in France.
The quality certification has been issued for the following category of action TRAINING ACTIVITIES.
This certification complies with the law of 5 September 2018 “for the freedom to choose one’s professional future”, which imposes mandatory certification according to a single national reference system. Training is a major stake for the quality assurance policies, particularly in the highly regulated sector of clinical research. It is also a key factor in maximising productivity. We provide an expert team to guide and train you.
Download our Qualiopi Certificate

Knowledge transfer
to be independent in designing and configure an eCRF and/or in configuring the related administrative environment. These training courses firstly target data managers and project managers.
End-users autonomy
enables investigators, CRAs (Clinical Research Assistants), CSTs (Clinical Studies Technicians), etc., to use the software features in an autonomous way.
This training activity is registered under number 11 92 19676 92. It is part of continuous training actions according to the articles L 6313-1 to L 6313-11 of the French Employment Code. It provides financial assistance in the name of continuous professional training.

2024 Key Figures
Number of training certificates issued :
132
Number of training
days completed :
32days
Overall trainee satisfaction rate :
97,2%
If you have any questions about our training program, please do not hesitate to contact our dedicated department by email at
formation-cleanweb@tentelemed.com
Download our Training’s Internal Rules
A LEADING POSITION AND
AN INTERNATIONAL DEPLOYMENT
64 +
countries
involved
20 000 +
investigational
Centres
3 million +
patients
in database
50 000 +
users
worlwide

LATEST NEWSOUR NEWS >
December 13, 2025
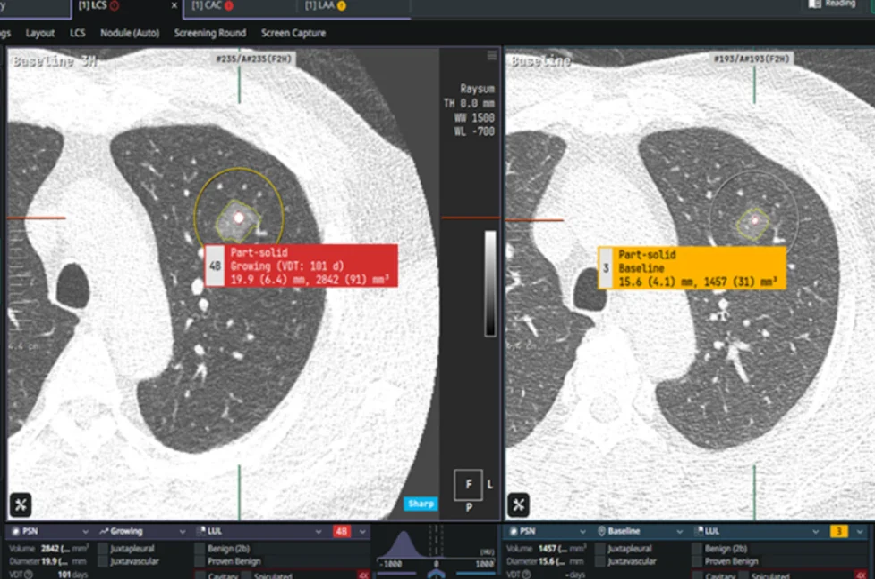
Mind Health With 53,000 new cases diagnosed each year, lung cancer is the deadliest cancer in France, according to data from the National Cancer Institute. Three-quarters of cases are detected at an advanced stage, which reduces the chances of recovery. The importance of screening is therefore crucial. In this field, the combination of low-dose CT […]
November 27, 2025
Med City News Healthcare quality isn’t improving at scale, despite advances in technology, because many systems treat it as another compliance task rather than a priority. Stephanie Mercado, CEO of the National Association for Healthcare Quality (NAHQ), argued that investing in workforce skills and standardizing roles can drive safer, higher-quality care while delivering measurable cost […]
November 27, 2025
Trial Site News Clinical trial reporting of treatment efficacy is the foundation of public trust in medicine. Yet pharmaceutical companies often report efficacy measured as a relative risk reduction (RRR) while omitting the more relevant absolute risk reduction (ARR). This statistical sleight of hand can make clinically insignificant benefits look spectacular! Consider a simple example A risk is […]