What role does AI play in lung cancer screening?
Mind Health
With 53,000 new cases diagnosed each year, lung cancer is the deadliest cancer in France, according to data from the National Cancer Institute. Three-quarters of cases are detected at an advanced stage, which reduces the chances of recovery. The importance of screening is therefore crucial. In this field, the combination of low-dose CT scans and artificial intelligence has proven its worth. How is this technology being integrated into screening strategies?
Large randomized multicenter studies conducted in Europe and the United States, such as NLST and NELSON, have demonstrated that structured screening for lung cancer using low-dose CT scans reduces lung cancer mortality by at least 20% in high-risk individuals. “The most established knowledge about cancer is the direct link between the stage of disease progression and the patient’s chances of recovery. All stage 1 cancers are curable. Advances in immunotherapy and targeted therapies remain marginal. Currently, screening programs are the main path to curing cancers,” said Fredrik Brag, CEO and co-founder of Median Technologies, during a press briefing in early November.
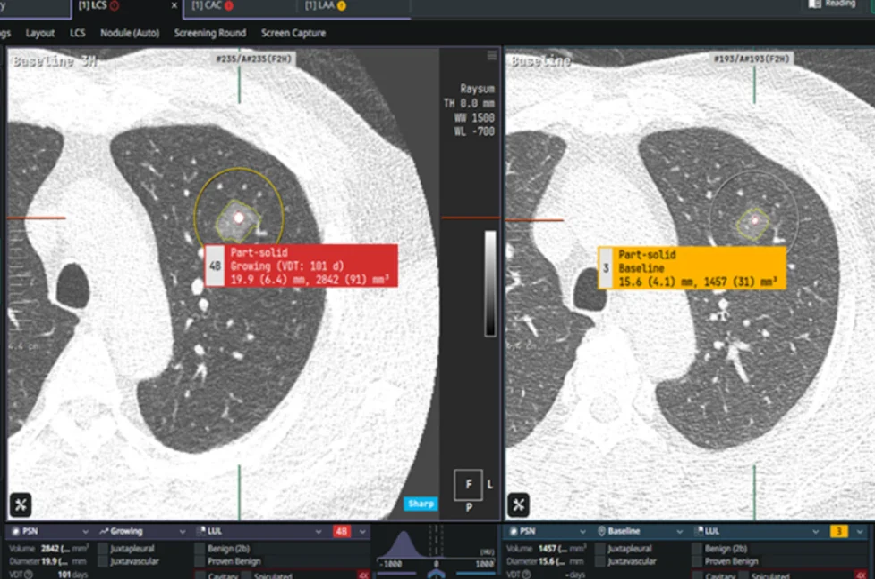
The French medtech company is entering the screening market with a software solution for lung cancer detection, eyonis LCS. This medical device, which is awaiting regulatory approval in the United States and Europe before commercialization, integrates both detection (CADe) and diagnostic (CADx) capabilities for pulmonary nodules.
Managing false positives: a key challenge
For Fredrik Brag, “the major challenge” of organized lung cancer screening lies in managing false positives. “Nearly one-third of patients over 50 have pulmonary nodules, which are often benign and not necessarily cancerous, such as calcifications,” he said. “Therefore, the ability to make a reliable diagnosis from the first exam is crucial to limit false positives, costs, and related anxiety.”
AI effectiveness proven
The AI model underlying Median Technologies’ solution was trained on a massive corpus of annotated data, including years of patient follow-up and treatment history. The technology has been clinically validated through two U.S.-based studies.
These findings align with the consensus among specialists that the effectiveness of low-dose CT scans combined with artificial intelligence is now beyond doubt. AI can help radiologists correlate images with diagnostic information. “Early studies demonstrating the medical benefits of screening relied on double human reading—a very labor-intensive process. Today’s deep learning-based AI solutions have advanced significantly and generate few false positives. Some countries, like Croatia and England, have already integrated AI to remove the need for a second human reader,” said Professor Marie-Pierre Revel (AP-HP), interviewed by Mind Health during JFR 2025.
For Fredrik Brag, “the biggest leap isn’t just in AI itself—sophisticated algorithms already existed twenty years ago. What has changed is computing power. Today, patient analysis happens almost in real time, allowing for continuous model iteration and performance improvement.”
Median Technologies achieves “98% accuracy in cancer detection and characterization,” according to Brag. Diagnostic evaluation relies on two indicators: sensitivity (the ability to correctly identify positive cases) and specificity (minimizing false positives). Determining a positive predictive value involves balancing these two. The risk, Brag summarized, is “overdiagnosis—detecting anomalies without understanding them.”
U.S. leads in organized screening
The United States is currently the most attractive market for AI-based lung cancer screening companies, as it is one of the first countries to implement an organized screening strategy. The annual low-dose CT scan program targets adults aged 50 to 80 with a history of smoking, based on USPSTF recommendations. “Currently, 15 million people are eligible for this screening, which is government-reimbursed,” noted Brag.
Since 2022, AI analysis of low-dose CT scans has been eligible for a $650 reimbursement code per scan, accessible to all U.S. imaging centers performing the test. This economic incentive appeals to companies like Median Technologies, which aims to enter the U.S. market—even though, for now, “only one incidental screening solution, by Oatmeal Health, benefits from this code,” Brag said.
Other foreign companies are also moving in. In September 2025, South Korea’s Coreline Soft signed a development agreement with Oatmeal Health to co-develop an integrated CADe–CADx product combining Coreline’s AI nodule detection engine with Oatmeal’s malignancy prediction algorithm.
Google Health has also ventured into lung cancer screening, though “the company has no ambition to develop medical devices,” according to Brag. Google began developing AI models for lung cancer detection in 2019, then published a retrospective U.S.–Japan study on how machine learning models can effectively communicate results to radiologists.
Median Technologies estimates the U.S. market for lung cancer screening at $2.9 billion annually. Brag hopes to achieve a rapid return on investment for the eyonis LCS device, which cost nearly €50 million to develop.
Pilot program launches in France
In France, a pilot lung cancer screening program called IMPULSION was unveiled in January by the INCa (National Cancer Institute). The research project, led by Prof. Marie-Pierre Revel (AP-HP) and Prof. Sébastien Couraud (HCL), aims to determine the optimal conditions for nationwide deployment. The first participants are expected to be enrolled by the end of the year.
On November 11, Coreline Soft was chosen as the exclusive AI software provider for the pilot. Its AVIEW platform will be deployed at over 100 sites, and Coreline will train users alongside the French Society of Radiology.
The INCa will fund the project with €6 million, and French Social Security will cover 100% of scan costs during the experiment. “Eligibility criteria, duration, frequency, number of images, radiological suspicion criteria, the contribution of AI, as well as the economic and organizational impact and effects on care access for other patients will be thoroughly analyzed. Our goal is to address the questions that hinder large-scale implementation,” said Prof. Norbert Ifrah, INCa President, at the program’s launch.
Screening programs worldwide
Low-dose CT screening programs are already in place in Croatia, Italy, England, and Australia. In Asia, screening initiatives have been introduced in Japan, South Korea, and China, each with its own model.
“In Japan, employers currently fund annual screenings for lung and breast cancer, with plans to extend coverage to other cancers. China is also making rapid progress. The absence of legacy infrastructure allows them to start from scratch and launch large-scale programs,” explained Fredrik Brag.
In Europe, Croatia was a pioneer, launching a national program in 2020. England followed, and Germany plans to launch its program in April 2026, with government criteria modeled after the NELSON trial.
